ByHeart Infant Formula went to 21 Countries. Are infants ill? Are they getting the BabyBig botulism anti-toxin?
The FDA has published the following warning:
Consumers worldwide should not use any ByHeart brand infant formula as all ByHeart products are included in this recall.
As we know, as of November 26, 2025, a total of 37 infants with suspected or confirmed infant botulism and confirmed exposure to ByHeart Whole Nutrition infant formula (various lots) have been reported from 17 states in the United States. For 36 cases with illness onset information available, illnesses started on dates ranging from August 9 to November 19, 2025. All 37 infants were hospitalized. No deaths have been reported to date.
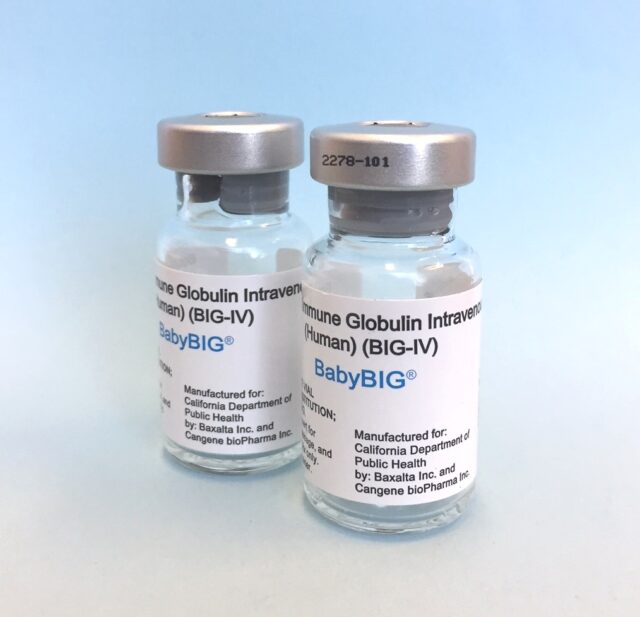
Treatment in the United States consists of supportive care and the used of BabyBIG (Botulism Immune Globulin Intravenous) which is an FDA-approved, human-derived antibody treatment for infant botulism types A and B. It consists of a purified immunoglobulin G (IgG) solution administered intravenously to neutralize the botulinum toxin. By providing antibodies against the toxin, BabyBIG can significantly shorten the length and reduce the severity of the illness, leading to shorter hospital stays and lower costs compared to untreated or delayed treatment cases.
As the FDA reported:
The ByHeart infant formula recall impacts markets outside the United States. Customer information provided by Amazon shows that a limited quantity of recalled ByHeart infant formula was distributed to Argentina, Brazil, Brunei, Canada, Chile, China, Colombia, Ecuador, Egypt, Hong Kong, Israel, Jamaica, Japan, Republic of Korea, Peru, Philippines, Romania, Singapore, South Africa, Thailand, and the British Virgin Islands.
However, is the botulism anti-toxin BabyBIG available to infants in foreign countries?
According to the Infant Botulism Treatment and Prevention Program (IBTPP), BabyBIG is not available through standard channels like pharmacies. Instead, it is an FDA-approved treatment for infant botulism that is only available through the IBTPP, which is run by the California Department of Public Health. A treating physician must contact the IBTPP’s on-call physicians for a clinical consultation and to initiate the process for obtaining the medication.
According to recent journal article, Human Botulism Immune Globulin Intravenous (BIG-IV) was licensed in the United States in October 2003 for the treatment of the rare, life-threatening infectious disease, infant botulism (IB) due to botulinum toxin types A or B. International use of BIG-IV began in July 2005 on a case-by-case basis following approval by the respective countries’ medicines regulatory agencies authorizing its importation. Since 2005, BIG-IV has been distributed to 16 countries on five continents.
Treatment of international patients was more likely to be delayed until laboratory confirmation was obtained. Longer time to BIG-IV infusion likely contributed to longer hospital LOS in international patients. Time to infusion differences could also be attributed to process delays in obtaining required approvals to import given BIG-IV is not licensed outside the U.S., transport challenges related to distance, and customs clearance delays.
Republished with permission from Bill Marler and Marler Clark. Copyright (c) Marler Clark LLP, PS. All rights reserved.
Source: https://www.marlerblog.com/case-news/byheart-infant-formula-went-to-21-countries-are-infants-ill-are-they-getting-the-babybig-botulism-anti-toxin/
Anyone can join.
Anyone can contribute.
Anyone can become informed about their world.
"United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
Before It’s News® is a community of individuals who report on what’s going on around them, from all around the world. Anyone can join. Anyone can contribute. Anyone can become informed about their world. "United We Stand" Click Here To Create Your Personal Citizen Journalist Account Today, Be Sure To Invite Your Friends.
LION'S MANE PRODUCT
Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules
Mushrooms are having a moment. One fabulous fungus in particular, lion’s mane, may help improve memory, depression and anxiety symptoms. They are also an excellent source of nutrients that show promise as a therapy for dementia, and other neurodegenerative diseases. If you’re living with anxiety or depression, you may be curious about all the therapy options out there — including the natural ones.Our Lion’s Mane WHOLE MIND Nootropic Blend has been formulated to utilize the potency of Lion’s mane but also include the benefits of four other Highly Beneficial Mushrooms. Synergistically, they work together to Build your health through improving cognitive function and immunity regardless of your age. Our Nootropic not only improves your Cognitive Function and Activates your Immune System, but it benefits growth of Essential Gut Flora, further enhancing your Vitality.
Our Formula includes: Lion’s Mane Mushrooms which Increase Brain Power through nerve growth, lessen anxiety, reduce depression, and improve concentration. Its an excellent adaptogen, promotes sleep and improves immunity. Shiitake Mushrooms which Fight cancer cells and infectious disease, boost the immune system, promotes brain function, and serves as a source of B vitamins. Maitake Mushrooms which regulate blood sugar levels of diabetics, reduce hypertension and boosts the immune system. Reishi Mushrooms which Fight inflammation, liver disease, fatigue, tumor growth and cancer. They Improve skin disorders and soothes digestive problems, stomach ulcers and leaky gut syndrome. Chaga Mushrooms which have anti-aging effects, boost immune function, improve stamina and athletic performance, even act as a natural aphrodisiac, fighting diabetes and improving liver function. Try Our Lion’s Mane WHOLE MIND Nootropic Blend 60 Capsules Today. Be 100% Satisfied or Receive a Full Money Back Guarantee. Order Yours Today by Following This Link.






